Last Updated on February 13, 2025
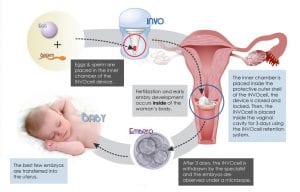
Fifteen years ago, the conceptwas devised but the technology was not there to support it. INVOcell is a gas permeable plastic capsule made in the in vitro fertilization (IVF) laboratory and contains a mixture of eggs and sperm. The eggs and sperm are collected in a manner similar to IVF; however, the eggs and sperm are not placed into a petri dish, under oil, or into an incubator. Instead, they are loaded into the capsule before fertilization results in an embryo. The INVOcell capsule is then placed in the posterior fornix of the vagina for 5 days so fertilization takes place in the patient’s body.
The INVOcell is a FDA-approved fertility treatment option, which means it has undergone extensive testing. A trial occurred where women were randomly selected for either an IVF or INVOcell treatment. The biggest difference was the number of eggs harvested, reflective of the amount of gonadotropins used.
Dr. Walmer shares more information on INVOcell with fertility patients in search of viable fertitly treatments and pathways. Listen now.
What to Expect
The invasive component of this outpatient process is one 5- to 10-minute procedure to harvest the eggs from the ovaries. The process is performed similarly to IVF. Before the harvesting of the eggs, the ovaries must be stimulated to produce follicles that hold the ripe eggs. The length of time for the stimulation cycle is the same in both IVF and INVOcell.
Before any medications are used, baseline ultrasounds are done to ensure no cysts are on the ovaries. Lower doses of medication are used for INVOcell compared to IVF. Monitoring is delayed until Day 10, and the decision to harvest the eggs is done on Day 10, 11, or 12.
The sperm are obtained before the eggs are harvested. During the egg harvest, the patient undergoes IV sedation. A needle is introduced through the back of the upper vagina and then into the ovaries for collection of the fluid that contains the eggs. The eggs are identified in the collected fluid by the embryologist. The eggs are then placed into the INVOcell capsule with the sperm within 10 to 20 minutes of the egg retrieval. The capsule is placed into the vagina during a pelvic exam with a speculum. A diaphragm device holds the capsule, which is pushed up toward the cervix to the top of the vagina and released.
The patient carries the capsule for 6 days before removal. She returns to the office for capsule retrieval. The embryologist analyzes the capsule to see which embryos developed. These are loaded into the transfer catheter and the embryos are placed into the uterus under ultrasound guidance, which is identical to the IVF procedure.
Candidates for INVOcell
INVOcell is useful in a group of women who are candidates for intrauterine insemination (IUI) or IVF. The key difference between INVOcell and IVF is that fertility doctors do not assist the actual fertilization process with INVOcell. The confidence level for the sperm fertilizing the egg is very high for INVOcell candidates.
An ICSI or intracytoplasmic sperm injection is not necessary as it is in IVF. Since the embryo forms in the INVOcell capsule, there are rarely issues related to an embryo forming. This process makes INVOcell more affordable by limiting the monitoring required for fertilization and the use of stimulation medications. It also means less eggs are harvested.
Differences Between IVF and INVOcell
INVOcell fertility treatment requires less medication and less stimulation of the ovaries and therefore less eggs are harvested. Additionally, the lab does not assist in the fertilization process with INVOcell. In IVF, the embryologist introduces the sperm to the egg to produce an embryo outside of the body. INVOcell occupies the space between ovulation induction, IUI, and IVF. In fact, it reduces the time for conception. In one study, 25 percent of women with unexplained infertility who underwent 3 IVF cycles became pregnant when an INVOcell capsule was used.
Additionally, the time to conception is reduced and a 55 percent pregnancy rate has been reported, which is comparable to up to 4 IUI cycles. In comparison with IUI, INVOcell has a higher success rate, no extensive monitoring, less expensive medications, and no expense for 5 days of culturing the embryo in the laboratory.
Clinical Trials and Risks
Many clinical trials support the safety of the INVOcell procedure. Safety concerns are similar to IVF and other fertility treatments. The risks must be weighed in relation to the desired goal of pregnancy. The biggest risk factor occurs after pregnancy, where physiological demands such as diabetes and hypertension may result from the huge fluid shifts occurring.
The physiology of the changes during a stimulation cycle are minimal compared to the risks associated with pregnancy. Ovarian hyperstimulation has traditionally been a risk in IVF, but this has changed because of the use of lupron triggers decreasing this risk. The INVOcell cycles are designed not to induce ovarian hyperstimulation, since lower dosages and reduced duration of medication is used.
Final Thoughts
INVOcell is an attractive fertility treatment option because the fertilization and the development of the embryo occurs inside the patient. The harvested eggs are out of the body for less than 20 minutes before being paired with the sperm in the capsule for fertilization. After fertilization, the embryos are out of the body for 5 to 10 minutes before being inserted into the uterus for implantation.

